

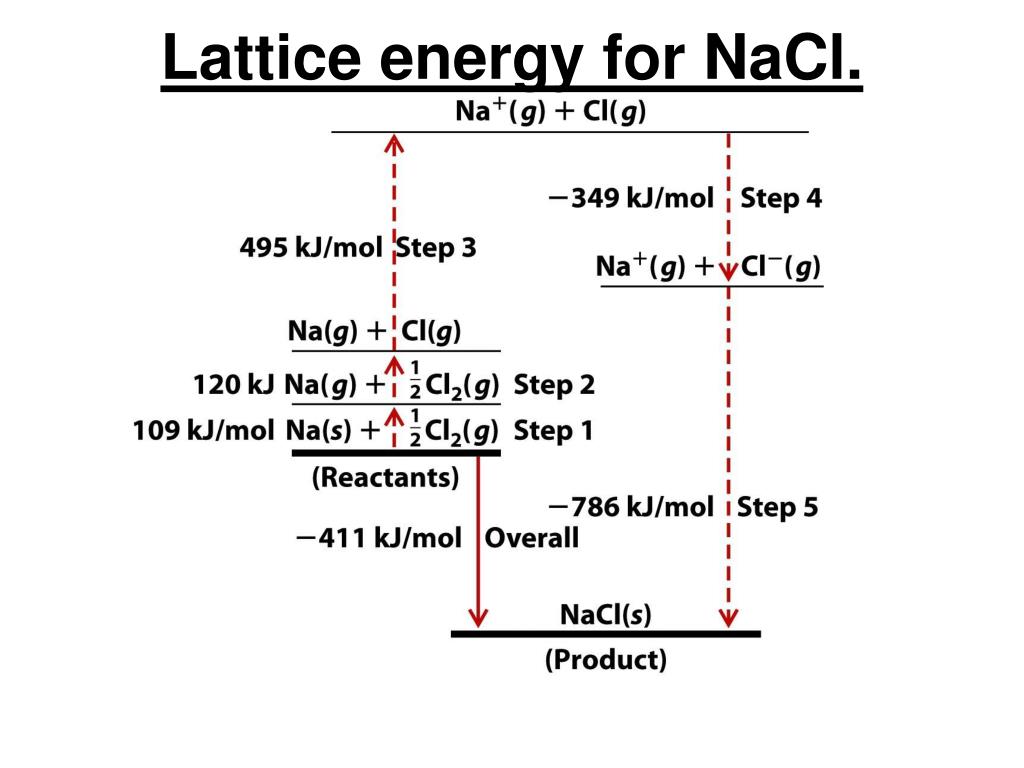
Polychromatic X-rays are to be directed against NaCl. Question: For Nat in the NaCl lattice, what is the distance to the nearest Nation 5.63 A 1.89 5.63 3.98 1.41 2.82 What is the sum total of all.
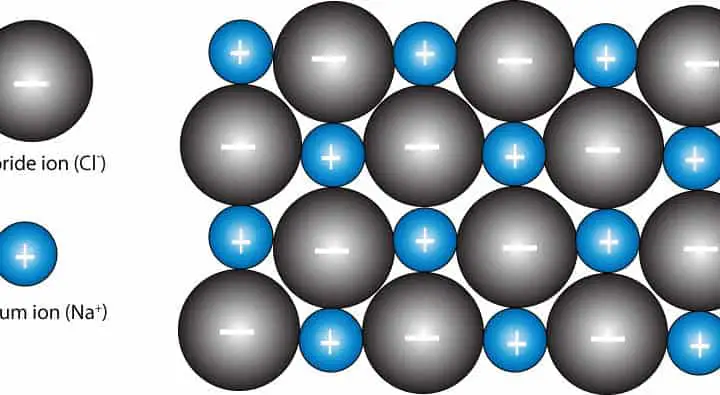
Occupy the octahedral sites in the Na sublattice. reciprocal lattice, Miller indices, Bragg scattering, atomic form factor, structure factor. The Na's occupy the octahedral sites in the Cl sublattice, and the Cl's Ions with a double charge produce lattices with a much. We can look at the NaCl as made up of fcc / ccp lattices interpenetrating. For example sodium chloride lattice enthalpy is given by: NaCl(s) Na+(g) + Cl-(g) H +790 kJ mol-1. Notice that there are 6 Cl surrounding the Na, and 6 Na around eachĬl. It may not be obvious, the red Cl's represent a different fcc / ccp unitĬell. There may be more than one way to divide a lattice into unit cells. The Cl sublattice must also be fcc / ccp. Since the repetition patterns of Na and Cl are the same in the lattice, If we compare this with the fcc / ccp unitĬell, it is clear that they are identical. horizontal vertical If we take the NaCl unit cell and remove all the red Cl ions, we are Click on the images below to view the open structure rotating. The same structure, but with the ions moved further apart allows NaCl is a crystal structure with a face centered cubic Bravais lattice and two atoms in the basis. However, the tightly-packed structures make it difficult to These stack so: Click on the images below to view the NaCl lattice structure Click on the unit cell above to view it rotating.
Sodium chloride also crystallizes in a cubic lattice, but withĪ different unit cell. Sodium Chloride crystal lattice SODIUM CHLORIDE


 0 kommentar(er)
0 kommentar(er)
